Preparation and Application of the Guanidine Carbonate
General Description
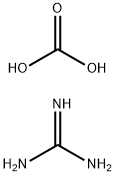
Guanidine carbonate, also known as carbonic acid aminourea. It can be dissolved in water and slightly dissolved in ethanol and acetone. It belongs to a strong organic alkali.It can be used in the preparation of sulfonamide drugs, cosmetics and dyes. In recent years, the foreign demand is very large, but there are only a few small-scale production in China.Therefore, there is an urgent demand to develop and produce it.[1]

Figure 1 Powder of Guanidine Carbonate
Preparation
Guanidine carbonate is obtained in 85.5% yield by treating the nitrate with NaOH in abs. alc., refluxing 1 hr., cooling to 10°, filtering off the NaNO3, satg. the filtrate with CO2 at 5℃, filtering off the NaHCO3, heating to 60℃ to decomp. the guanidine bicarbonate to CO2 and Guanidine carbonate, and filtering off the Guanidine carbonate which ppts. Alternatively, the satn. may be carried out at 60-65℃, but the purity of the product drops to 93% from 96%.[2]Production of guanidine carbonate from Dicyandiamidincarbonat by cooking the aqueous Lsg., characterised in. that the cooking with continuous introduction of CO2 takes place.It is thus obtained without any cleavage to urea, CO2 and NH3 quantitatively pure guanidine carbonate. Time script formation is a 20 to 25°/0ig. Dicyandiamidincarbonatlsg with continuous feeding of CO2 and simultaneous concentration approximately 5-6 hours cooked. The result is an approximately 50%.[3]
In addition, production of guanidine carbonate from aqueous solutions of other guanidine salts by double conversion, characterised in. that, in these solutions NH3 in the shot to the binding of the required carbonate quantity required, further (NH4)2CO3 or NH4HCO3 approximately in the formation of guanidine carbonate theoret. required amount is introduced and the auskrist. Salt is separated off, wherein the supply amount of NH3 and carbonate are such that after transient Auflsg. of added carbonate with which I no (NH4) 2CO3 fails.[4]
Application
The effect of guanidinc carbonate on tobacco necrosis virus (TNV). 0.05M Guanidine carbonate, completely prevented TKV infection in the intact primary leaves attached to the plant of P. vulgaris var. Saxa after being introduced or absorbed into the leaf tissue either with the virus or within 4 hours after inoculation. Outside the leaf tissue, i.e. in vitro, the chemical did not affect virus infectivity. Results also indicate that a pH of 10.5 and the carbonate of the guanidine carbonate did not inhibit the virus. The sole activity was, perhaps, contained in the guanidine base. The inhibitory effect of the chemical, however, depended mainly upon the extent of introduction of it into the leaf the issue, as one or two proper applications with a brush were sufficient for virus inhibit,ion whereas spraying once or twice was less effective. After spraying, the chemical was perhaps, not sufficiently absorbed into the leaf tissue to render it completely resistant to virus infection.[5]
Guanidine carbonate has been used as a complexing agent to polish TaN/Ta and polysilicon films.It was found that the guanidine carbonate in presence of oxidizer and silica abrasives can enhance the RRs of both types of Ru films by forming Ru oxide-guanidinium complexes which can be easily polished/ removed by silica abrasives.[6]Guanidine carbonate serves as an effective surface-complexing agent for such chemical mechanical planarization applications, where the rate of Ta removal can be chemically controlled through pH-tuned selectivity with respect to the removal of Cu lines. The surfacemodifying roles of Guanidine carbonate make it can be used as an attractive complexing agent for Ta chemical mechanical planarization by employing electrochemical techniques in previous study work. In additon, The bicarbonate/carbonate anions of Guanidine carbonate also facilitate Ta removal through the generation of ion-incorporated tantalum pentoxide.[7]
Reference
[1]W Z. Production and application of guanidine carbonate[J]. Friend of Chemical Industry, 2001, 000(005):35-35.
[2]Desseigne G. Preparation of guanidine carbonate[J].Bulletin de la Societe Chimique de France.1955,1193-4.
[3]Mayen H. Production of guanidine carbonate (Herstellung von Guanidincarbonat)[P]. DD 458437.1928,4,12.
[4]Grosskinsky O, Klempt W, et al. Production of guanidine carbonate (Herstellung von Guanidincarbonat)[P].DE 1008727.1957,05,23.
[5]Varma J P.Inhibition of tobacco necrosis virus by guanidine carbonate[J].Virology 1968, 36(2):305-8.
[6]Amanapu H P, Sagi K V, Teugels L G, et al. Role of guanidine carbonate and crystal orientation on chemical mechanical polishing of ruthenium films[J]. ECS Journal of Solid State Science and Technology, 2013, 2(11): P445.
[7]Rock S.E.Material characterization in the electro-analytic approach for applications in chemical mechanical planarization and electrochemical energy systems[J].Dissertations Theses Global.2015,04,20.
You may like
Related articles And Qustion
See also
Lastest Price from Guanidine carbonate manufacturers

US $5.00-0.50/KG2025-05-13
- CAS:
- 593-85-1
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 593-85-1
- Min. Order:
- 1KG
- Purity:
- 99.0%min
- Supply Ability:
- 10 TONS